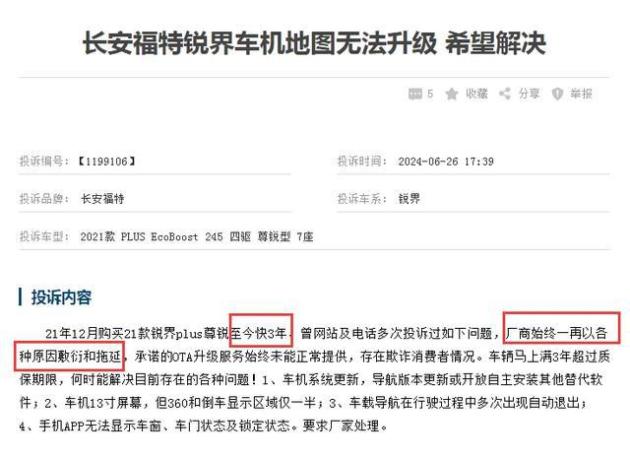
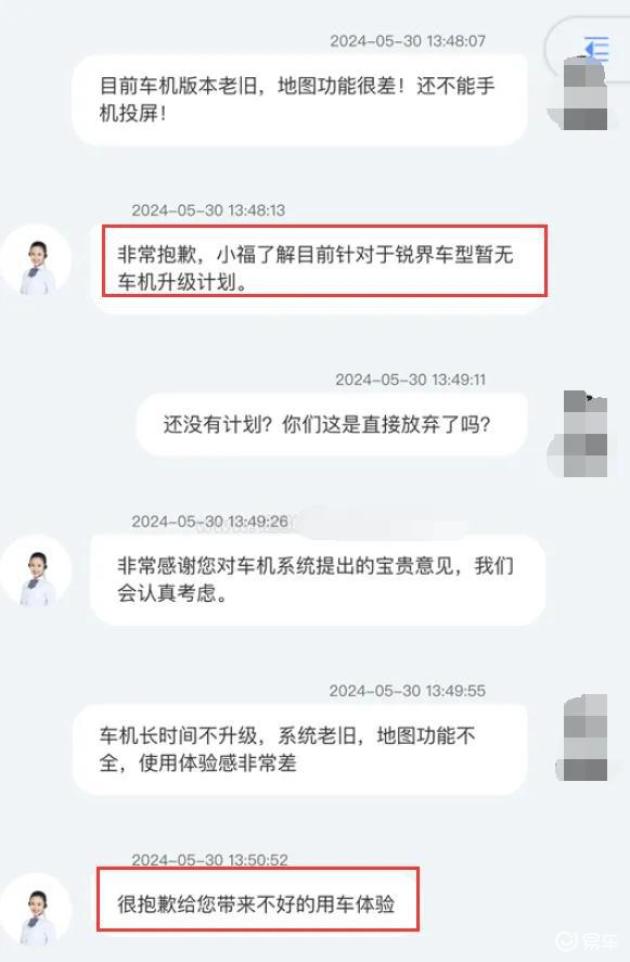
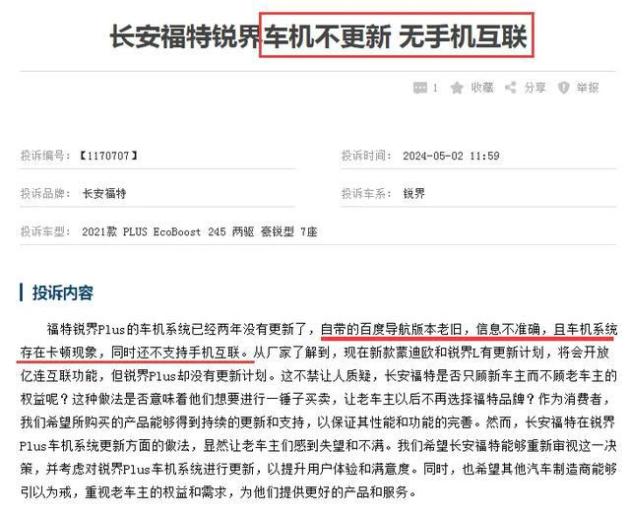
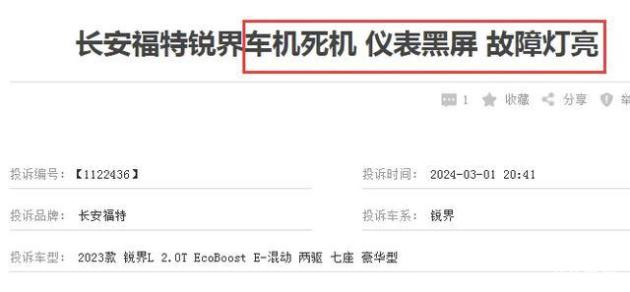
In order to standardize and guide the R&D, production and registration of specific human immunoglobulin, and further clarify the technical evaluation standards, under the deployment of National Medical Products Administration, the Drug Testing Center organized and formulated the Technical Guiding Principles for Pharmaceutical Research and Evaluation of Specific Human Immunoglobulin (see Annex).
According to the requirements of the Notice of the General Department of National Medical Products Administration on Printing and Distributing the Release Procedures of Drug Technical Guidelines (No.9 [2020] of the General Administration of Drug Administration), with the approval of National Medical Products Administration, it is hereby promulgated and shall come into force as of the date of promulgation.
This is for your information.
Appendix: Technical Guiding Principles for Pharmaceutical Research and Evaluation of Specific Human Immunoglobulin
National Medical Products Administration yaoshen center
May 20, 2022
I. Overview
Specific human immunoglobulin (HIG) is a highly effective immunoglobulin preparation prepared from plasma containing specific antibodies.
The sources of plasma include plasma with high titer antibody after the patient recovered from infection with a pathogen, and plasma containing specific antibodies by hyperimmune injection of healthy blood donors, that is, injection of vaccines or other antigens to make the injected people produce antibodies. Specific human immunoglobulin (hereinafter referred to as "special immune product") is an indispensable blood product because it contains specific antibodies with high titer, and its effectiveness in preventing and treating specific diseases is better than that of ordinary human immunoglobulin.
In order to encourage, standardize and guide the research and development of special exemption products, this guiding principle is formulated with reference to the relevant technical requirements at home and abroad and combined with the actual situation in China. This guiding principle is only based on the current technical development and scientific cognition, aiming at the general pharmaceutical research technical requirements of special exemption products. The applicant/holder can also adopt other more effective methods and means according to the actual situation of the research and development of the exempted products, but it should conform to the law of drug research and development and provide scientific and reasonable basis to ensure the safety, effectiveness and controllable quality of the products.
Second, the scope of application
These guiding principles are applicable to the special immunity products made of specific antibody plasma obtained by direct screening of specific antibody of plasma donors and active immunization. They have similar product characteristics and pharmacological characteristics, and their pharmaceutical requirements are both common and different. At present, the varieties included in People’s Republic of China (PRC) Pharmacopoeia (hereinafter referred to as China Pharmacopoeia) include hepatitis B human immunoglobulin, rabies human immunoglobulin, tetanus human immunoglobulin, etc. The research and development and registration of special exemption products at home and abroad are shown in Schedule 1, and the modes of administration are divided into intravenous injection, intramuscular injection and subcutaneous injection.
III. General principles
1. Security.
Because of the particularity of blood products, there is a potential risk of virus pollution, and virus safety control is the core content of blood products quality control. The collection, inspection, storage, transportation, traceability and the immune requirements of plasma donors of the plasma exempted products shall conform to the provisions of "Plasma for Blood Products Production" in China Pharmacopoeia and "Quality Management Standard for Plasma Collection Stations". It is suggested that screening research on B19 and other pathogens should be carried out according to product characteristics and clinical users, especially some special varieties (such as anti-D immunoglobulin). It is suggested that the applicant/holder cooperate with relevant departments to carry out epidemiological monitoring in the area where the plasma station is located. Actively promote the detection technology of nucleic acid in raw plasma and mixed plasma, and formulate standards and requirements. Special attention should be paid to the epidemic situation and detection methods of new and sudden infectious diseases.
2. Specific antibody screening.
The accuracy and reliability of specific antibody screening results is the key to ensure the quality of raw plasma. In the case of large-scale production, the amount of single plasma detection involved is large, so it is necessary to establish a suitable antibody titer detection method and fully verify it by the method in the early stage of research and development. Single plasma can be screened for specific antibodies by immunolabeling method, and the correlation between binding titer and neutralization titer can be established. The combined plasma, stock solution and preparation shall be tested for specific antibodies by the method specified in China Pharmacopoeia or the verified method. Usually, based on virus neutralization test (mouse neutralization test, micro-cell lesion count and plaque reduction test), immunolabeling test (enzyme labeling, fluorescence labeling, isotope labeling, etc.), hemagglutination inhibition test, immunodiffusion test, immunoturbidimetry test or other immune/biochemical tests, the research is carried out by quantitative and semi-quantitative methods based on reference materials.
3. Production technology
The process development of this kind of products can refer to the process of similar products that have been listed, and platform technology can be used. The use of chromatography is encouraged to improve the purity of products, but the selection of chromatographic parameters and the influence of the quality of chromatographic resin on the quality of products should be fully considered, and the inactivation and removal ability of viruses by the process should be paid special attention to. The verification of virus inactivation/removal should meet the requirements of General Principles of China Pharmacopoeia "Virus Safety Control of Biological Products", "Technical Methods and Verification Guidelines for Virus Removal/Inactivation of Blood Products" and technical documents such as ICH and WHO.
Management rules and operating procedures should be established for personnel safety protection and wastes in the production process, bioactive substances and viruses should be inactivated according to proven inactivation treatment methods, and effective protective measures should be taken to protect operators to avoid risks such as virus transmission.
Four, pharmaceutical research and evaluation points
(1) Raw plasma and materials for production
1, plasma source and quality control
Quality control of single plasma: single plasma must meet the relevant quality control requirements issued by the national health administrative department. Raw plasma used for production must have legal sources (plasma station certification documents, etc.) and pass inspection and quarantine, and should have complete reagent data (marketing certification documents, etc.) and screening records (antigens/antibodies/nucleic acids) for screening blood-borne infection markers.
Immune procedure: immune procedure and dosage of antigen used are the key factors to determine the concentration and titer of antibody in plasma. It is necessary to define the immunization methods. For example, when active immunization is carried out with approved vaccines or other antigens, these immunogens should have complete source certification information and batch information. The immunization program should refer to the requirements of vaccine instructions, or be formulated according to the immunogenicity of antigen and the reactivity of plasma donors, but the specific immunization program adopted should prove its safety. Subjects should have full right to know, and at the same time make corresponding countermeasures for possible problems.
Quality control of mixed plasma: A certain amount of plasma should be mixed, which should meet the requirements of China Pharmacopoeia in combination with product characteristics. At present, ordinary people are required to mix the plasma of at least 1000 plasma donors. Specially exempted products generally require plasma mixing from at least 100 plasma donors, except for some special varieties (such as anti-D immunoglobulin products).
No matter the plasma obtained by active immunization with approved vaccine or immunogen or the plasma immunized after natural infection, the specific antibody content standard of each plasma and combined plasma should be formulated at the time of IND declaration. The plasma whose antibody level is up to the standard is used for plasma production. For varieties that have not been recorded in China Pharmacopoeia, there should be a clear basis and a proven detection method for determining the titer of specific antibodies in raw plasma and preparations. When NDA is declared for the varieties included in China Pharmacopoeia, the antibody titer standard should not be lower than the requirements of China Pharmacopoeia.
2. Raw materials for production
Strict quality control of raw materials used in the production of special exemption products is a necessary measure to reduce the risk of contamination by exogenous factors or toxic impurities in products and avoid the activation of other factors in plasma. In addition to clear sources, quality standards and inspection reports, raw materials for production should also focus on the use of animal-derived raw materials (such as heparin) and avoid using animal-derived raw materials as much as possible. TSE/BSE risk assessment should be conducted for raw materials used in production.
When using filter AIDS (perlite, diatomaceous earth, etc.), filter membranes and filter element materials in the separation process, it is necessary to establish appropriate quality control standards according to the requirements of different process stages, control the introduction risk of exogenous factors, control bacterial endotoxin and heavy metals, and formulate reasonable limit requirements. In addition, it is necessary to pay attention to the risk of residues that may be mixed into the final product and potentially harmful to human body, and control them if necessary.
(2) Production technology
1. Stock solution
The initial raw material of the special immunity products is raw plasma or plasma components. The modified Cohn method or KistlerNitschmann method can be used to separate the special immunity products, and other methods can also be used for separation and purification, such as octanoate precipitation and chromatography.
The separation and purification process adopted should ensure that the physicochemical and biological properties of the product and the biological activity of the Fc segment of IgG are well preserved. The production process must avoid or eliminate the pollution of microorganisms (bacteria, viruses) and their metabolites (pyrogens) to the maximum extent. It is necessary to strengthen the control of production process and consider the influence of the removal of filter AIDS and the selection of filter media on the products. It should be paid attention to that IgG fractions obtained by different separation methods may contain higher levels of IgA and IgM. At the same time, attention should be paid to the rationality of sterilization and filtration steps, and the integrity of the filtered membrane should be checked.
It is necessary to specify the amount of slurry for pre-clinical, clinical samples and market-scale samples. Generally, continuous production should be carried out, and the production capacity of each process step should be matched with each other, and the processing should be completed at one time. In each process step, it is the key to determine the process control parameters and scope and meet the requirements. When using ethanol and other precipitation methods, it is necessary to control the quality of ethanol and other reagents, and study the protein concentration, temperature, pH value, ionic strength, treatment time and so on, so as to define the acceptable limit range. In the process of dissolution and precipitation of plasma components, avoiding IgG coprecipitation is the key to improve the product yield, especially the key indexes such as temperature and adding time should be fully studied to investigate the influence on the key quality indexes such as protein purity and molecular size distribution. When chromatographic method is used, it is necessary to optimize chromatographic conditions and determine reasonable parameters according to the nature and concentration of protein in the product, such as the capacity of chromatographic column, ionic strength of feed solution and buffer solution, pH value of buffer solution, flow rate, contact time and temperature, etc. The determination of these parameters should be based on the research data of process development, and limit standards and allowable ranges should be formulated. At the same time, it is necessary to study the cleaning and regeneration (service life) of chromatographic resin, the load of chromatographic column and the extract, especially the use of chromatographic packing with potentially harmful ligands. It is necessary to pay attention to the volume, protein concentration, recovery rate and electrophoretic purity of each separation and purification step, and evaluate the process-related impurities (such as ethanol, sedimentation aid, IgA, aluminum, heavy metal ions, etc.) and product-related impurities.(such as polymers, etc.). If necessary, in order to prove the ability of the process to remove impurities, it may be necessary to use standard addition test to evaluate the ability of the process to remove some potential pollutants. If operations that may affect product quality, such as temporary storage and transportation of intermediate products, need to be supported by necessary stability research data.
Encourage the use of innovative and improved processes (including the improvement of ethanol separation process) to improve the utilization rate of plasma and improve the quality of special exemption products. In the early stage of process development, attention should be paid to the identification of key process parameters, the integrity of process control in different separation stages and the rationality of process parameter setting. If the applicant/holder has already marketed products with the same separation and purification process, and the existing process research data and control parameters can be used for reference, the process verification of three batches of new special exemption products should be carried out.
Considering the preciousness of plasma resources, the pilot-scale research data suitable for clinical trials can also be used for the declaration of IND stage. However, when applying for NDA, it is necessary to have complete commercial scale research data.
2. Preparation
Clarify single prescription and batch prescription, and explain the basis of rationality of preparation prescription. Auxiliary materials suitable for medical use should be used, and the stability of the supply chain should be ensured. For accessories from multiple suppliers, it is necessary to carry out full research. For brand-new excipients that have not been used in similar preparations at home and abroad, a comprehensive study should be carried out and related declarations should be made. No bacteriostatic agent shall be added to the special exemption products for intravenous injection. Because some cosolvents (such as polysorbate 80) have the potential risk of causing allergic reaction and hemolysis reaction, safety evaluation should be carried out. The preparation process (such as stirring speed) should be studied. In principle, the preparation of semi-finished products should come from a batch of stock solutions. If different batches of stock solutions are used to prepare semi-finished products, the possible risks should be evaluated.
The temperature of products, the duration of packaging, the temperature and humidity of packaging environment should be controlled. Special exemption products involving freeze-drying technology should have data such as freeze-drying process parameters and freeze-drying curves verified by commercial batches, indicating the influence of freeze-drying technology on product quality, especially on biological activity.
3. Process verification
For the products exempt from intramuscular injection, it is necessary to determine the process flow and key process parameters before NDA declaration, and continuously produce three batches of products to verify the stability of the process, so as to provide a supporting basis for the products to go on the market. For the special exemption products for intravenous injection, the process flow and key process parameters should be determined as far as possible before the confirmatory clinical trial, and the stability of the process should be verified by continuous production of three batches. If there are differences between clinical trial samples and commercial scale batches in terms of production technology or site, it is suggested to carry out change research, and it is suggested to carry out research and exploration on the worst process conditions at an appropriate stage, and it is necessary to prove that qualified products can be produced under the determined process parameters and quality control limits. If multiple production sites or production lines are involved, verification research should be carried out.
(III) Virus safety and virus removal/inactivation verification
The detection of single plasma and mixed plasma should meet the requirements of China Pharmacopoeia, and the number of cases of mixed plasma should meet the sensitivity requirements of the kit/detection method. For anti-D immunoglobulin, the nucleic acid load of B19 virus in plasma should be ensured to be less than <104 IU/ml.
Because of the differences in the ability of inactivation/removal of lipid-coated and non-coated viruses by various methods, it is recommended to use two virus inactivation/removal methods with different mechanisms to carry out verification, and to clarify the technical conditions and control parameters of virus inactivation/removal process steps, including product uniformity, upper and lower temperature limits, inactivation time, filtration pressure/speed/temperature/solution composition/membrane integrity, etc. Attention should be paid to the rationality of setting the position of deactivation/removal process steps. When selecting virus removal/inactivation method, the effect of virus inactivation/removal should be balanced with product activity and general safety.
It is necessary to consider the potential interaction between inactivation steps, the influence of inactivation process on antibody integrity and clinical efficacy, whether new immunogens are formed and the risk of toxic residues that may be introduced. If the production process changes, which may affect the virus removal ability, it needs to be verified again. In addition, the samples used for inspection should be representative of the process. With the development of technology, some new methods of virus inactivation can also be applied in special exemption products, which need to be proved to be scientific and effective and fully verified.
If the products with the same process steps have been approved and registered, and the virus inactivation/removal process has been verified, the newly declared products will only change the mode of administration, and the products with the same production equipment, process and operation as the original products in commercial batches can not be subjected to virus inactivation/removal process repeatedly on the premise of meeting the relevant technical requirements such as Technical Methods and Verification Guidelines for Removing/Inactivating Viruses from Blood Products and Technical Guidelines for Pharmaceutical Change Research of Listed Biological Products.
In order to ensure the virus safety of the final product, we should consider the setting of plasma station from epidemiological data, strictly select plasma donors, screen plasma and manage the quarantine period, optimize the production process and add virus removal/inactivation steps, and evaluate the virus removal ability of the whole process steps of producing products. To evaluate the virus inactivation/removal ability of the production process and the maximum load of specific virus that may exist in the initial plasma, and to ensure the virus safety of the product to the greatest extent, it is necessary to ensure that the verification includes the worst case.
(D) Quality research and control
1. Analysis of physical and chemical characteristics
It is necessary to gradually improve the analysis of physical and chemical characteristics in combination with product types, preparation types and drug development stages. IND declaration can be made based on the preliminary research data of physical and chemical characteristics, and NDA declaration should be made by comparing the physical and chemical characteristics, including antibody integrity, antibody subtype distribution, circular dichroism analysis, etc.
2. Biological activity
Biological activity research should be fully carried out, and it is suggested to study the correlation between binding titer and neutralization titer detection methods. From the aspects of supporting the rationality of the process and considering the influence of the process on the product, it is suggested to establish a method for detecting the biological function of Fc, and to study the sialic acid content of Fc segment in the development stage. Production technology, antibody titer, IgG polymer, IgG subclass, etc. will affect the results of biological function test of Fc. In addition, it is suggested to analyze the binding ability of Fc segment to immune cell surface receptors, the activation of complement function by Fc segment, and the regulation of phagocytosis.
3. Purity and impurities
Attention should be paid to the components that affect the function of this kind of products, and the purity is generally not less than 95%. In addition to carrying out research on impurity residues and proving the ability of the process to remove impurities, the impurity level limits of batches at different stages (if there are clinical and commercial batches) should be set reasonably. It is suggested that a sensitive method should be used to control the content of impurity protein.
Polymers produced during the preparation of special immunity products are related to anti-complement activity (ACA), and the content of IgG polymers should be controlled. In order to prevent patients with congenital IgA deficiency from causing serious allergic reaction after using special immunity products, the content of IgA should be controlled. The content of anti-A and anti-B hemagglutinin should be studied and the limit standard should be established to prevent hemolytic reaction caused by large amount of infusion. Encourage the detection of coagulation activator levels and establish standards. It is suggested that anti-D antibody detection should be carried out for intravenous special immunity products from special plasma sources. In addition, in order to prevent the risk of thrombosis, it is suggested to control the FXIa content of intravenous special immunity products and establish standards.
Encourage the detection of other miscellaneous proteins in blood, such as kallikrein activator, immunoglobulin M, transferrin, albumin, α1- antitrypsin, α2 macroglobulin, haptoglobin, α1- acidic glycoprotein, ceruloplasmin, fibrinogen, antithrombin III, plasminogen, fibronectin and C1 esterase inhibitor.
Special exemption products, especially those for intravenous injection, should also focus on the control of insoluble particles. Insoluble particles can not be metabolized in human body, and it is easy to induce thrombosis if they stay in tiny blood vessels. The content of aluminum should be controlled. Aluminum has potential risks to the central nervous system, bones and other organs, and this risk is related to the infusion dose.
4, quality analysis and standards
The listed special exemption products should meet the requirements of China Pharmacopoeia, and the rationality of testing items should be fully considered for new special exemption products. Quality standards should be set reasonably according to the characteristics of products with different administration methods, such as intravenous special exemption products, and FXIa factor, aluminum ion and IgA content should be increased.
When applying for IND, special attention should be paid to antibody titer, harmful residues and other related testing items.
When applying for NDA, the quality standards of stock solution, semi-finished products (if any) and preparation should be reasonably drawn up based on the data of various research and development stages and stability inspection, on the basis of statistical analysis of multiple batches, combined with the requirements of China Pharmacopoeia and the situation of similar products, with emphasis on the quality control of potency, and more stringent internal control standards should be encouraged. Advanced and mature methods should be used for titer detection, and various complementary analysis methods should be encouraged for quality control in the early stage of research and development. If auxiliary materials such as maltose, polysorbate 80 and glycine are added to the preparation, it should be controlled in the quality standard of the preparation and its content range should be defined.
5, analysis method and methodology verification
Combined with the characteristics of products, select a reasonable analysis method and carry out sufficient methodological verification to ensure the feasibility of the method.
At the time of IND declaration, at least the preliminary verification of antibody titer and harmful residue detection method should be completed.
When applying for NDA, a complete analysis method should be established and verified. Clarify the accuracy, precision (including repeatability, intermediate precision and reproducibility), specificity, detection limit, quantitative limit, linearity, range and durability of the determination method. Methods other than those in China Pharmacopoeia should be fully verified when applying for NDA. If the determination method is changed greatly, the scope of re-verification should be determined according to the degree of revision of the method.
Establishment and verification of antibody titer detection methods: research on product-specific antibody titer detection methods and standards should be carried out before research and development. It is suggested to study the correlation between binding titer and neutralization titer detection methods. Try to use the method of China Pharmacopoeia, and try to choose the classic and industry-standard method for those not included in China Pharmacopoeia. Clarify the test operation flow, test parameters, preparation and calibration of work standards and criteria for judging results. According to the antibody titer detection method itself, select appropriate indicators to carry out methodological verification and establish verification scheme. Generally speaking, in terms of linearity, the correlation coefficient of neutralization titer determination method should meet the pre-set acceptable standards. In terms of accuracy, the ratio between the actual titer of neutralizing antibody and the theoretical titer of serum with different dilution times is 1, or the slope of regression line in covariance analysis is parallel. In terms of precision, the coefficient of variation should be controlled within 30% as far as possible. In terms of durability, it is necessary to investigate the effects of cell density (generation), neutralization virus dose, neutralization reaction conditions and time, and different positions of orifice plates on the test results. In order to study the influence of possible degradation products and degradation pathways on titer determination, the samples can be destroyed by accelerated methods such as strong light irradiation, high temperature and high humidity. Encourage the use of working standards as quality control, reduce test interference factors, and improve the accuracy and reliability of results.
Verification of detection methods for hazardous residues: Usually, hazardous residues are at trace level, and the sensitivity of detection methods determines the reliability of the results. Encourage the use of more advanced methods and instruments with higher sensitivity based on the methods in China Pharmacopoeia, and pay attention to distinguish the detection limits of instruments and methods. The obtained detection limit and quantitative limit data must be verified by samples with similar contents.
say
Test process and detection limit results, including accuracy and precision verification data. The linear indexes such as recovery rate (%) and relative standard deviation (RSD%) should be listed as regression equation, correlation coefficient, residual square sum linear graph, etc. The range should be drawn up within 20% of the specified limit according to the preliminary measured data, and in general, various methods with different principles should be used to verify each other. Methodological verification of product-related impurities: Methodological verification shall be carried out according to the requirements of China Pharmacopoeia. If the commercial kit is used for residue detection, it is suggested to study the selection of detection kit (such as IgA detection), and the detection kit should explain the sensitivity, specificity, detection limit, quantitative limit and linear range. In general, the accuracy, precision, linear range, interference test, reference interval and other indicators of the kit should be verified. The variation coefficient of precision should be within 20%, the accuracy deviation should not exceed 10%, the linear range determination coefficient (R 2) should be ≥ 0.98, and the relative deviation of interference test should be less than 10%. Attention should be paid to identify the factors that affect the accuracy of the determination results during the release test of products, such as pH value, PK activity, ionic strength of product solution, reaction temperature, etc., which will affect the determination results of PKA, and low pH conditions may reduce the PKA activity of products.
6, reference (standard)
The quality control reference of antibody titer should be established according to the requirements of relevant guiding principles. When applying for NDA, it is necessary to clarify the key information such as the source and quality control of reference materials, and the reference materials should be related to the samples used in clinical experiments, paying attention to the accuracy and traceability of assignment. If international/national standards are adopted, the information of standards used (source, batch, etc.) shall be specified. If it is a self-made reference, the preparation process, calibration and stability should be studied.
(5) Stability study
Stability test runs through the whole product life cycle and is the basis for formulating product standards and expiration dates. The stability test should be carried out in accordance with the China Pharmacopoeia and the relevant guiding principles of stability research of biological products at home and abroad. In the declaration stage of IND, preliminary stability inspection can be conducted to ensure the quality of samples in clinical stage. Complete a comprehensive stability inspection before NDA declaration, select suitable packaging materials, define storage and transportation conditions, and formulate a reasonable validity period. The investigation of influencing factor test and accelerated test should be studied as far as possible until the product is unqualified. Long-term stability investigation should extend the observation time of products as much as possible. In general, the liquid dosage form should be placed upside down and upright for stability test. Products with different closed systems should be tested for stability respectively. If a new production site is introduced to produce intermediate products, unless there are other reasons, the stability research of intermediate products and finished products should be carried out. The activity of Fc segment is an important indicator of the effectiveness of this kind of products, so it is suggested to investigate the related stability. For the special exemption products in liquid dosage form, the thermal stability test should be carried out, and the visible foreign bodies should be checked. The results should meet the requirements of China Pharmacopoeia.
(6) Packaging and sealing container system
The source and control standard of inner packaging materials should be clarified, and the compatibility and sealing of packaging materials should be studied in accordance with the relevant guiding principles such as Technical Guiding Principles for Compatibility Research of Chemical Injection with Medical Glass Packaging Container (Trial Implementation), Technical Guiding Principles for Compatibility Research of Chemical Injection with Plastic Packaging Materials (Trial Implementation) and the requirements of ICH related guidelines. Extracts, dissolved substances, sloughs, etc. shall meet the limit requirements. Pay attention to the influence of the extract of inner packing rubber stopper and glass bottle on the preparation.
V. Explanation of nouns
1. Blood Products refer to therapeutic products derived from human blood or plasma, such as human albumin, human immunoglobulin, human coagulation factor, etc.
2. Human Normal Immunoglobulin (HNIG): also known as gamma globulin or multivalent immunoglobulin, is an immunoglobulin preparation separated from healthy human plasma by low-temperature ethanol protein separation method or other approved protein separation methods, including intravenous immunoglobulin, intramuscular immunoglobulin and subcutaneous immunoglobulin.
3. Specific human immunoglobulin (HIG): It is a high titer immunoglobulin preparation prepared from plasma containing specific antibodies. The sources of plasma include plasma with high titer antibody after the patient recovered from infection with a pathogen, and plasma containing specific antibodies by hyperimmune injection of healthy blood donors, that is, injection of vaccines or other antigens to make the injected people produce antibodies. Different from ordinary immunoglobulins, such preparations must have at least one high titer antibody for the prevention and treatment of specific diseases in clinic.
4. Anti-complement Activity (ACA): A substance in serum or tissue fluid that can nonspecific bind to complement and make it inactive, such as protease and ester.
VI. References (omitted)